|

Are you looking for a doctor who accepts Medicaid in Las Vegas? Nearly a million citizens are currently enrolled in Nevada’s Medicaid and CHIP program. It can be challenging to find a provider that accepts Medicaid. Luckily, My Virtual Physician is approved to accept Medicaid as payment for medical services, including doctor’s appointments. Patients can visit online or connect virtually with doctors at our physical hybrid location in Las Vegas, located at 2217 Paradise Road. Read more below to find out how to see a Medicaid doctor in our Las Vegas clinic (or online).
My Virtual Physician has a new physical location on Paradise Road in Las Vegas. At this hybrid location, patients looking for a Medicaid doctor in Vegas can have their vitals taken, get lab samples taken, and connect virtually with a doctor in a private room through our videoconferencing setup.
Whether you just need to get a quick covid test, physical exam, or get a prescription refill, our hybrid clinic in Las Vegas is set up to handle your basic medical needs. We have primary care physicians as well as specialists on staff to meet with you today. Our Nevada specialists include:
Read more about our doctors and which states they are licensed to practice in.
Check the clinic’s current hours here before you visit. As you walk into our clinic, our medical assistant will make you comfortable and take your vital signs for the doctor’s review. You’ll be guided to a private room to meet virtually with one of our doctors via our secure videoconferencing setup. Upon leaving, if you were prescribed any medications, you can either head to the pharmacy or head home to wait on your order-by-mail prescription. We cater to meet patient needs, so just let us know which pharmacy option you prefer during your visit.
If you prefer to schedule an appointment time rather than wait during a walk-in visit, My Virtual Physician welcomes you to reserve an appointment time by filling out the form on this page.
You have options to either book an appointment for a virtual visit on your own equipment (smartphone or computer) from any location in the state or at our physical hybrid location at 2217 Paradise Road in Nevada. Both ways to see your doctor are covered by Medicaid.
If you schedule an appointment time at our hybrid clinic in Las Vegas, your experience will be similar to the walk-in option above. Our medical assistant will welcome you, collect any samples and vitals as needed, and show you to a secure room where you’ll visit privately online with one of our doctors.
A third way to see a Medicaid doctor in Las Vegas is through online appointments. While you’ll need to be a resident of Nevada for the visit to be covered by Nevada Medicaid, you may still be covered by your state’s Medicaid plan in other regions. Check our coverage map and payment page to see if your state’s Medicaid plan is included in our coverage area.
Currently, other states with Medicaid coverage with My Virtual Physician include: Arizona, Colorado, Tennessee, and New York.
To schedule your online appointment today, click below.
Book Appointment Now Call For An Appointment
Regardless of which part of the state you’re located in, if you prefer not to physically travel to our hybrid clinic, you don’t have to. If you have a smartphone or computer that can record video and sound, then you can meet with our Medicaid doctors through a telemedicine visit online.
The process works as easily as this:
Today, getting medical care is simple and easy. You can quickly find doctors in Las Vegas who take Medicaid. And care is available through a variety of channels, including in-person, hybrid clinics, or 100% virtually. My Virtual Physician is here to help make healthcare more accessible to those who need it, including those who have Medicaid.

Getting sick in Vegas is not fun! If you’re in the big city, whether on business, pleasure, or because you live here, you may be looking for a quick way to get tested to rule out Covid-19.
While the drive-through Covid testing sites have shut down, the government has suspended the free at-home test program, and testing requirements for Covid-19 have dropped off, My Virtual Physician aims to provide easy access to Covid testing for those who want to be checked.
With the recent opening of its Hybrid Clinic, located at 2217 Paradise Road in Las Vegas, you can now walk-in to get your Covid testing done right on the Vegas strip.
My Virtual Physician’s hybrid clinic in Las Vegas offers three Covid testing options for your choosing. We can do:
PCR tests are most accurate, but they should be avoided if you tested positive within 90 days since they can still detect prior viral genetic material for that length of time.
Antigen tests are not as reliable and may require multiple tests to confirm results. The CDC recommends repeating this test after 48 hours to ensure an accurate reading.
At-home tests can be purchased over the counter or picked up at our hybrid clinic in Las Vegas. These are antigen tests and can give you results in under 30 minutes from the comfort of your own home. These are ideal if you are picking the test up for someone who prefers to stay at home.
The CDC has updated the Covid-19 testing information and now recommends that you get tested if you have symptoms. Common Covid-19 symptoms include:
Before you visit someone who is at a high risk of severe Covid-19, such as the elderly or immunocompromised, get a Covid test.
If you’re looking to treat your covid-19 symptoms, My Virtual Physician can help with that as well. Our clinic doors are open if you’re in town and need to get tested and consult virtually with our doctors. Treatment must begin within a few days (five to seven days, depending on treatment) after you first have symptoms and is reserved for those who are at higher risk of becoming very sick. Treatment reduces your risk of hospitalization or death from Covid-19.
If you’re not eligible for Covid-19 treatment, our doctors can help you manage your symptoms with a care plan tailored to your experience. Check your eligibility by comparing your situation to the criteria below:
Our physicians can help determine whether you would benefit from Covid-19 treatment, and if so, prescribe the appropriate treatment. Come see us today at our Covid testing site in Las Vegas or schedule your appointment online to address your symptoms and get treatment after a positive at-home test result.
Book Appointment Now Call For An Appointment
Ending Covid-19 Isolation
A friend messaged me the other day to inquire about a positive rapid antigen test (RAT). She tested positive for Covid nine days prior and had diligently isolated herself in a bedroom, away from her family. She re-tested on day 5, day 6, and day 7: still positive. And now, again, the ‘test’ line on her RAT test was positive on day 8. “Does the positive test mean I should keep isolating? And why is the isolation period five days if people really stay infectious longer?”, she asked.
The CDC revised its isolation guidelines in December 2021, stating that most people who test positive for Covid but have been fever-free for at least 24 hours can stop isolating after five days (with day 0 being the start of symptoms or, if asymptomatic, the date of the positive test) and did not need a negative coronavirus test to leave isolation. They do still recommend, though, that people continue taking precautions such as mask-wearing and refraining from travel on days 6 - 10.
A CDC study showed that up to half of people infected with Covid will continue to be infectious on days 6 - 9. Thus, although it is not mandatory, many people who have the option to are continuing to isolate until they receive a negative rapid antigen test (RAT). RAT tests correlate well with the amount of virus in the body and how likely you are to spread the virus to others.
It is important to remember that - in contrast to RATs - while polymerase chain reaction (PCR) tests are considered the gold standard for the initial diagnosis of Covid, they are not helpful in determining when to end isolation because they can remain positive for weeks to months after an infection, well after a person has fully recovered and is no longer contagious.
Although it may have been more in line with the viral timeline to continue recommending ten days of isolation, it is likely that the CDC recognized the burden the longer isolation period carried, with the greatest toll falling on the most vulnerable sectors. In fact, despite current CDC recommendations, Amazon just recently announced it would be ending its Covid-19 paid leave policy; it’s likely other large employers will soon follow suit.
If you have been looking forward to a new kind of COVID-19 test, one which doesn’t involve any sort of implement being scrubbed around inside your nasal passages, you can stop holding your breath. In mid-April, the Food and Drug Administration granted emergency use authorization (EUA) to the InspectIR COVID-19 breathalyzer test. But don’t toss those rapid antigen tests (RATs) just yet.
The initial utility of the breath test is limited. The breathalyzer machine itself is about the size of a piece of carry-on luggage and requires trained personnel to supervise its operation. Therefore, while it will be a welcome addition to the existing rapid diagnostic tests in health care settings and mobile test sites, it won’t be for home use.
Now that they have received FDA authorization, InspectIR Systems is expected to bring their COVID-19 breathalyzers to market in ~ 10-12 weeks and will produce 100 devices per week. Each device can run approximately 160 samples per day. In the company’s study of ~ 2,400 asymptomatic people, the breathalyzer correctly identified 91% of positive infections and 99% of negative infections (PCR was used as the gold standard).
The sample—a breath of air—is blown into a straw attached to an analyzer called a gas chromatograph-mass spectrometer (GC-MS). Within 3 minutes, the GC-MS can identify whether the breath contains a mixture of volatile organic compounds (VOCs) which are associated with SARS-CoV-2 infection. It’s important to note that certain foods and substances can affect the breath test; the testing instructions specify to avoid eating, drinking, and tobacco products in the 15 minutes prior to taking the test. The FDA recommends positive breath tests be confirmed with a molecular test.
No doubt more breath tests are on the way to development and approval. It remains to be seen how they will change the COVID-19 testing landscape.
The other day I went online to order an at-home COVID-19 rapid antigen test (RAT). The number of options was mind-boggling! How are people supposed to choose? Are they just picking the cheapest test? Or the one at the top of the list?
Unfortunately, not all rapid antigen tests (RATs) are created equal. Although the FDA has not yet granted full approval to any at-home RATs, many have undergone an expedited review and subsequently received emergency use authorization (EUA).
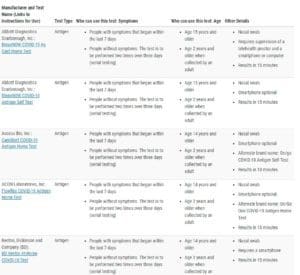
The key takeaway as you scroll through the myriad options is to simply verify that the test which makes it into your shopping cart has also made it onto the Federal Drug Administration (FDA) updated list of authorized diagnostic tests: In Vitro Diagnostics EUAs - Antigen Diagnostic Tests for SARS-CoV-2 | FDA.
The FDA’s list currently has seventeen antigen tests that have received EUA, listed in alphabetical order by manufacturer. If you click on the highlighted link for a test (on the left side of the screen) you will be linked to home use instructions for that test. Other information on the list includes the test type (antigen or molecular), when to use the test (for example, if you’ve experienced symptoms within the last 7 days), eligible age range, whether or not a smartphone is required to view the results, the type of sample (eg nasal swab), and how long it takes for test results to be available.
Reassuringly, the top three at-home rapid antigen COVID-19 tests at both Amazon (BinaxNOW, iHealth COVID-19 Rapid Test and BD Veritor) and CVS (iHealth, BinaxNOW, and FlowFlex) are all FDA authorized.
A test's accuracy can wane as new SARS-CoV-2 variants emerge. Therefore, it is important to continue to check the FDA’s updated site to make sure that the test you are ordering has received authorization or approval.

Amazon at-home COVID-19 tests (screenshot; not inclusive) FDA's list of authorized at-Home over-the-counter COVID-19 diagnostic tests (screenshot; not inclusive)
First off, a disclaimer; anyone reading this will surely know that our understanding of long COVID is in its nascency. Many of these answers are a ‘best guess’ based on today’s data and will doubtless change over time as more information becomes available.
What is long covid and how is it diagnosed?:
While there is widespread agreement that Post-Acute Sequelae of SARS-CoV-2 infection (PASC), or ‘long covid’, consists of ongoing symptoms beyond what would typically be expected after recovering from Covid, experts vary in the duration that qualifies as long covid, with a range between ~2-3 months of from the onset of COVID-19. There are no specific tests to determine whether a person has long covid, thus the diagnosis is clinical.
What are the three most common symptoms of long covid?
Less common:
Chest pain, anxiety, depression, difficulty speaking, muscle aches, loss of smell and/or taste
Duration:
Variable, typically ~ several weeks - 6 months (up to 9 months and longer reported)
How likely is it that a person will develop long covid after an initial infection?
Debatable; percentages vary from single digits up to ~ 50%.
Does anything increase the risk of long covid?
Studies suggest that older people, those with a more serious initial Covid-19 infection and/or those with certain underlying comorbid conditions (such as chronic lung disease, diabetes, and heart disease) may be at greater risk of developing long covid. There is also some evidence that women may be more prone to long covid, and that vaccination may confer a degree of protection against long covid, but this data is still emerging.
How is long covid treated?
Because long covid can involve many different body systems, a multidisciplinary approach and a range of treatments directed toward the specific body systems affected are key. Although it can be tempting to rush back to normal activities, post-exertional malaise–meaning profound fatigue with overexertion–is common. Rehabilitation can help direct a gradual return to activity and promote recovery.
You’ve had a cough and sniffles for over a week. COVID-19 rapid antigen and PCR tests were negative. What else might be causing your symptoms?
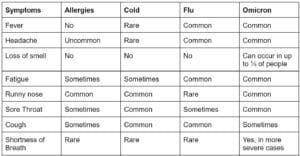
Although we’ve all become acutely aware of SARS-CoV-2, the coronavirus which causes COVID-19, other coronaviruses have been with us throughout human history, often presenting as common colds. Symptoms of COVID-19 overlap with colds, the flu, and even seasonal allergies, and differentiating between the myriad causes of a respiratory illness can be challenging. Shared symptoms include runny nose, fatigue, cough, headache, and sore throat. Loss of taste or smell, however, is unique to COVID-19 and is atypical of other respiratory illnesses.
Respiratory viruses such as the flu tend to be seasonal, peaking in the winter in the northern hemisphere (September to April). In people who are ill enough to require hospitalization, respiratory panels are run which include multiple pathogens, such as influenza, adenovirus, parainfluenza, respiratory syncytial virus (RSV), human metapneumovirus, and rhinovirus. However, in adults not sick enough to require hospitalization, the main viruses of concern are influenza and SARS-CoV-2.
Influenza, ‘the flu’, usually presents with a rapid onset of fever, cough, and body aches and should be suspected when there is an outbreak in the community. Similar to COVID-19, the preferred test is a molecular assay (nucleic acid amplification test), which can detect very small amounts of virus and is highly sensitive and specific, meaning it has a low percentage of incorrect test results. The conventional reverse transcription polymerase chain reaction (RT-PCR) assay takes ~ 1-8 hours and can distinguish between influenza A and B and subtypes of A. Rapid molecular tests can distinguish between influenza A and B but cannot differentiate subtypes of A. Rapid molecular influenza tests are more practical in an urgent care or emergency setting as results are back in ~ 15-30 minutes. Finally, multiplex RT-PCR are tests that can simultaneously check for many respiratory influenza, SARS-CoV-2, and bacterial pneumonia, among others.
Below is a table outlining common differences between allergies, a cold, the flu, and the Omicron variant of SARS-CoV-2. As new variants emerge, symptoms of COVID-19 will continue to evolve. If your symptoms are worsening and you have concerns, please contact your healthcare provider for advice.
Rapid Antigen COVID-19 Tests: Update on Authorized and Unauthorized Tests
Rapid antigen tests (RATs) provide a cost-effective and convenient way to screen for COVID-19 in the comfort of your own home. A simple kit, complete with a nasal swab, solution, and easy-to-read result tray, can provide a quick ‘yes’ or ‘no’ when you are wondering if your sniffles are due to seasonal allergies or the latest variant of SARS-CoV-2 (the virus which causes COVID-19). As such, RATs have proved a valuable tool for managing our new so-called pandemic life.
In addition to the eight free rapid antigen tests (RATs) available from the government, RATs can be purchased over the counter at local or online pharmacies or retailers such as Walgreens, CVS, Target, Amazon, or Wal-Mart.
The U.S. Food and Drug Administration (FDA) has continued to grant emergency use authorization of rapid antigen tests for COVID-19 as new data emerges. Recently, several RATs have been authorized; conversely, several have had their letters of emergency use authorization revoked.
FDA list of currently approved RATs:
FDA list of RATs that have had their emergency use authorizations revoked (no longer authorized):
Recall that rapid antigen tests may be negative very early on in an infection. In the case of exposure to COVID-19, it is recommended to check a RAT 2-3 days after the exposure (if you remain asymptomatic) and then continue to test every day or every other day for six days hence. If you have been exposed to COVID-19 and are experiencing symptoms, a negative RAT test may be a false negative and should be followed by a PCR (if available).
The Cost of COVID-19 Testing:
To date, the U.S. government has funded a huge COVID-19 relief effort, including covering the cost of medically indicated COVID-19 testing for Americans with and without health insurance under the CARES Act and the Families First Coronavirus Response Act, respectively. In March 2022, however, the House passed a federal spending bill that omitted ongoing funding for the program which pays for uninsured individuals to obtain COVID-19 testing, treatment, and vaccinations. The White House is working to secure additional funding, but if the program ends it will mean the end of free testing and treatments for millions of Americans.
The burden of COVID-19 testing costs, however, isn’t limited to the uninsured. Individuals with insurance may need to self-pay for COVID-19 testing if it is needed for a non-medical indication, such as travel or a return-work program, neither of which are included in the insurance coverage mandate.
So exactly what is the cost of a COVID-19 test?
The answer is that the cost varies hugely, even for the exact same type of test.
Consumer Reports recently investigated the out-of-pocket cost of COVID-19 tests. One of the issues they found is that testing manufacturers and labs are allowed to set their own prices and insurance providers are required to cover testing costs at the price set by the company. In addition, the number of FDA-approved tests is fairly limited, meaning that demand has consistently outstripped supply during COVID-19 surges. This combination has allowed companies to engage in price gouging, preying on desperate consumers.
A study by America’s Health Insurance Plans in July 2021 found that “On average, a COVID-19 test in the commercial market costs $130. In contrast, out-of-network test providers charged significantly higher (more than $185) prices for more than half (54%) of COVID-19 tests in March 2021—a 12% increase since the beginning of the pandemic. ”
More recently, in December 2021, Mira (a low-cost health care coverage provider) reported on a survey they conducted of the top twenty-three urgent care facilities in the United States to determine the out-of-pocket cost of COVID-19 diagnostic tests. They found that “the average reported cost of a PCR test is $137, and the average cost of a rapid antigen test is $189. Overall, most urgent care clinics offer diagnostic testing at a price between $100-$200.”
In addition to the above, the availability of at-home tests has increased and may provide a lower-cost option if paying out of pocket. Rapid antigen tests (RATS) typically run ~$10 per test. Three at-home molecular tests are currently FDA approved for emergency use and do not require a prescription. These are more sensitive for detecting early COVID-19 infection but are considerably more expensive, ~$75-100 on average.
Bottom line: When purchasing a COVID-19 test, consider what type of test is best suited to your needs and then do a brief cost comparison before paying top dollar.
What is a rapid test?: An antigen-based COVID-19 test, often called a rapid test, can be purchased over the counter (online or in a pharmacy) and taken at home. It works by searching for protein pieces of the COVID-19 virus. Depending on the test, either a sample from the nose or mouth is obtained via a swab, then placed into a solution. In most kits, you put a few drops of the solution plus sample into a test disc and wait ~ 15 minutes. If the test is positive, there will be two-colored lines, the control line and the test line. If the test is negative, only the control line will be positive.
It is important to note that rapid antigen tests are less sensitive than polymerase chain reaction (PCR) tests which amplify even very tiny amounts of viral genetic material and thus rapid tests might be negative very early on in an infection. Also, whereas a PCR test can stay positive for weeks to months, even after you are no longer contagious, a rapid test will become negative once the virus count has dropped below the level of infectivity.
What does it mean if a test has been ‘authorized’, ‘unauthorized’, ‘approved’ or ‘unapproved’?: One of the responsibilities of the Federal Drug Administration (FDA) is ensuring that vaccines and medical devices are safe and effective. In order for a medical device, such as a COVID-19 test, to be deemed safe and effective it must undergo an extensive review. This time-consuming process is required for full FDA approval. If a product is needed urgently, though, it may undergo an expedited review; those that pass this more rapid review receive what is called an emergency use authorization (EUA).
Currently, none of the COVID-19 rapid antigen tests have received full FDA approval, but many have been authorized for emergency use. The FDA maintains an updated list of antigen and molecular diagnostic tests for SARS-CoV-2 which have received EUA: In Vitro Diagnostics EUAs - Antigen Diagnostic Tests for SARS-CoV-2 | FDA.
It is particularly important to check the list of authorized products if you are buying a rapid test online, as the internet is rife with fraudulent and unauthorized tests. Most recently, on March 1st 2022, the FDA issues safety notices warning against use of unauthorized rapid tests made by ACON Laboratories, SD Biosensor and Celltrion.
How much does a rapid test cost?: The out-of-pocket cost of rapid antigen COVID-19 tests varies, averaging ~ $15-20 per kit (two tests per kit). Thankfully, the government has ramped up the accessibility of free rapid COVID-19 tests. In January 2022, all US households became eligible to request a one-time order of four free rapid COVID-19 tests, either via the website COVIDTests.gov or by calling 800-232-0233. At his State of the Union address, President Biden announced that Americans would be able to receive a second set of free rapid tests; four more per household for those who already ordered one set and eight tests (two sets) for those who haven't yet ordered. Private health insurers are also mandated to cover up to eight at-home antigen tests per month, per covered individual, with reimbursement capped at $12 per testing kit (two tests per kit). The insurance provider will usually cover the upfront cost for in-network pharmacies and retroactively reimburse members who buy test kits from out-of-network pharmacies. Original Medicare beneficiaries will be able to receive up to 8 free over-the-counter COVID-19 tests in the early spring of 2022. In the meantime, they can obtain free at-home tests from community health centers and Medicare-certified health clinics.
Non-oral COVID-19 Treatment Options in the Outpatient Setting
The proliferation of treatment options for SARS-CoV-2 can be seen as one of the success stories of the global COVID-19 pandemic.
Individuals who have been diagnosed with COVID-19 and are at high risk of hospitalization may be eligible for treatment with non-oral medications which have received FDA approval or emergency use authorization (EUA). These medications are administered intravenously (IV), typically in an infusion center. They can be divided into two categories: 1) monoclonal antibodies and 2) antiviral drugs.
Antibody Therapy:
Monoclonal antibodies are synthetic versions of antibodies made by the immune system against SARS-CoV-2. The body’s immune system typically takes a couple of weeks to build up antibodies against SARS-CoV-2 after an exposure (either via infection with the virus or via vaccination), therefore the administration of synthetic antibodies can effectively fast-track this targeted immune defense. Antibody treatments work by binding to certain key parts of the virus, blocking the virus from binding to and entering cells.
Antibody treatments with EUA for outpatient use include sotrovimab and bebtelovimab.
Antiviral Therapy:
Remdesivir is an antiviral drug that was previously authorized for use in hospital patients, but just recently (end of January 2022) received FDA authorization for outpatient use. Remdesivir works by incorporating into the viral RNA (ribonucleic acid), inhibiting the ability of SARS-CoV-2 to replicate.
What Are the Treatment Criteria?:
All three of the above medications are approved for use in adult or pediatric (age 12 yo and older, weighing ≥ 40 kg) patients who have mild-to-moderate symptomatic COVID-19 and who are at high risk of progression to hospitalization. In order to be efficacious, they should be started as soon as possible, no later than 7 days after symptoms began.
How Well Do The Treatments Work?
Studies have demonstrated > 80% efficacy of both remdesivir and sotrovimab for preventing hospitalization or death due to COVID-19 (if started with 7 days of symptom onset). Data are still emerging for bebtelovimab; preliminary studies have also shown a reduction in hospitalization or death in high-risk patients.
What Are the Common Side Effects?:
Monoclonal antibody treatments for COVID-19 are generally well tolerated. The most commonly reported side effects are injection site reactions and infusion-related reactions (1% of patients who received sotrovimab experienced infusion-related reactions).
The most common side effects following administration of Remdesivir were nausea, headaches, and rash. Elevated liver enzymes (a laboratory finding) are common in patients who receive Remdesivir, and caution is advised in those individuals with liver or kidney disease.
Two years into the global pandemic, antiviral pills have emerged as a new therapeutic option. In late December of 2021, the FDA gave emergency use authorization (EUA) to two oral antiviral treatments for COVID-19: Paxlovid (Pfizer Inc.) and molnupiravir (Merk & Co). These hotly anticipated medications are intended for use in newly infected individuals who are at high risk of developing severe disease.
How do they work?
Paxlovid is a combination of two different medications, nirmatrelvir and ritonavir. Paxlovid blocks a key type of enzyme, called a protease, which cuts up SARS-CoV-2 (the virus which causes COVID-19) proteins into their active forms. When the protease is blocked, the virus can’t replicate (~90% of viral replication is inhibited), and the infection slows down, allowing the immune system to ramp up and fight the remaining virus.
Molnupiravir is incorporated into the RNA (the genetic blueprint) of SARS-CoV-2, resulting in defective genetic material and effectively stopping the virus from multiplying.
When and in whom can they be used?
Both pills are indicated for use in patients who are covid positive, symptomatic with mild to moderate disease, and at high risk of progression to severe disease. The treatments work best when started as soon as possible after a COVID-19 diagnosis and they must be started within five days of symptom onset. They are administered twice daily for five days.
Paxlovid: authorized for use in all adults and in children who are 12 years or older and weigh at least 88 pounds. There is a high potential for drug interactions (eg statins, blood thinners) with Paxlovid; make sure to inform your health care provider which other medications and over-the-counter supplements you are taking. Paxlovid may not be appropriate for individuals with liver disease, kidney disease, or those infected with the HIV virus
Molnupiravir: authorized for use in adults 18 or older. Cannot be used in pregnant or breastfeeding women. Molnupiravir is only approved for use in those “...for whom alternative COVID-19 treatment options authorized by the FDA are not accessible or clinically appropriate”.
How WELL do the treatments work?
In the studies cited by the FDA, Paxlovid reduced the rate of COVID-19 related hospital admission or death by ~88% and molnupiravir by ~ 33%.
What are the most common side effects?
Paxlovid: altered taste or loss of taste, diarrhea, high blood pressure, muscle aches
Molnupiravir: diarrhea, nausea, dizziness
* If you have recently been diagnosed with COVID-19, developed symptoms within the past five days, and think you may be eligible for one of the oral antiviral medications (eg you are at high risk of severe disease), please reach out to your health care provider.
Ongoing supply shortages of COVID-19 tests have spawned a new online market for scammers: fake COVID-19 tests.
Shady companies are taking advantage of increased consumer demand to sell faux at-home test kits online.
If you do unwittingly use a fake COVID-19 test and receive a false negative result, you risk spreading the infection to others and not receiving appropriate treatment.
The counterfeit COVID-19 tests are widespread enough that the Federal Trade Commission published a statement in January warning consumers of the issue and advising them of steps to take to avoid the fake tests.
A legitimate COVID-19 test is one that has been authorized by the FDA. The FDA maintains an updated list of antigen and molecular diagnostic tests for SARS-CoV-2 (the virus which causes COVID-19) which have received emergency use authorization (EUA). If you are buying an at-home test kit online and want to make sure it is legit, you can check the list: In Vitro Diagnostics EUAs - Antigen Diagnostic Tests for SARS-CoV-2 | FDA.
The FDA also has a list of fraudulent COVID-19 products you can search: Fraudulent Coronavirus Disease 2019 (COVID-19) Products | FDA
Additional steps you can take to avoid illegitimate COVID-19 test kits:
The new year found Americans mired in the peak of yet another SARS-CoV-2 wave, with the preceding holiday season scarred by overstretched health care systems and testing capacity. In response, the Biden administration has increased the accessibility of at-home COVID-19 tests on multiple fronts.
In January 2022, all US households became eligible to request four free rapid COVID-19 tests either via the website, COVIDTests.gov, or by calling 800-232-0233.
At the same time, the government also mandated that private health insurers cover up to eight at-home antigen tests per month, per covered individual, with reimbursement capped at $12 per testing kit (two tests per kit). Out of pocket costs vary between ~$10-25 per kit. Typically, the insurance provider will cover the upfront cost for in-network pharmacies and retroactively reimburse members who buy test kits from out-of-network pharmacies.
Although Medicare was not initially included in the coverage mandate, at the beginning of February the Biden administration announced that Medicare beneficiaries, including Medicare Advantage, would also become eligible for eight free at-home COVID-19 antigen tests per month, beginning in the early spring.
The expanded coverage will include over 60 million Americans, many of whom fall into the higher risk health category.
Medicaid recipients already have full coverage of at-home COVID-19 tests (8 per month).
At the most basic level, there are two categories of COVID-19 tests; a diagnostic test and an antibody test. A diagnostic test can diagnose a current infection, whereas an antibody test can tell you whether your immune system has been exposed to COVID-19 (either via infection or vaccine) in the past. An antibody test cannot be used to diagnose a current infection.
Diagnostic tests include PCR (polymerase chain reaction) and antigen tests.
PCR is considered the “gold standard” for diagnosing infection with COVID-19. It is a molecular test that looks for COVID-19’s genetic material (RNA). PCR is very sensitive, meaning that almost everyone who actually has a COVID-19 infection will have a positive test. PCR tests are analyzed in a lab and are most often performed in a health care setting, such as a clinic, doctors’ office, pharmacy, or designated testing center. If performed at home, the sample kit will need to be mailed to a lab for analysis. The samples are most commonly collected via a nasopharyngeal swab (the swab is inserted into the nostril and up toward the back of the nose) or a salivary sample. Result turnaround can vary from ~ 1-7 days. Due to the labor of the processing, they are fairly expensive, around $150 (without insurance coverage). During COVID-19 peaks, PCR results have often been quite delayed when testing capacity has not been able to meet high demand.
Antigen tests have the advantage of speed, with results available in ~ 15 minutes. Instead of genetic material, antigen tests search for protein pieces from the COVID-19 virus. They can be purchased over the counter, are performed at home via a nasal swab, and are less expensive than PCR tests, with prices running around $20-24 per kit (two tests per kit). Antigen tests are similar in design and appearance to a home pregnancy test, with a colored line designating ‘positive’ or ‘negative’ showing in the box at the top once the sample is finished processing. Antigen tests are less sensitive than PCR tests and may be negative early on in an infection.
If you are experiencing symptoms of COVID-19 and have a negative antigen test, it is recommended to obtain a PCR test and continue to isolate until the result of the PCR test is known (if results are delayed, then continue to isolate for 5 days at minimum, with symptoms resolving and 24 hours free of fever prior to ending isolation; continue wearing a well-fitting mask for an additional five more days after the isolation period has ended).
Because PCR tests are so sensitive, they can remain positive for up to twelve weeks after a person has recovered from COVID-19 and is no longer contagious. If you have been diagnosed with COVID-19 and subsequently recovered, an antigen test is the best test to determine if you are still shedding enough virus to transmit COVID-19 to other people.
Many labs are experiencing long delays –5 days or more– in processing COVID-19 PCR tests.
If this is the case for you, and you are wondering what to do while awaiting your test, you can utilize serial at-home tests (typically rapid antigen tests), if you have access to them. These tests come back in ~ 15 minutes, but they are less sensitive for testing small amounts of the virus and will be negative very early on in the infection. One approach might be to begin testing with antigen tests on day 3 (day 0 is your exposure), then test daily while awaiting the PCR result.
If you are asymptomatic, never develop symptoms, and antigen tests remain negative 5 days apart (eg day 3 and day 8), it is unlikely you have COVID-19.
If you have or develop symptoms of COVID-19, and you are not able to obtain antigen tests OR even if the initial antigen test was negative, assume you likely have COVID-19 and isolate accordingly.
The table below is a basic outline of recommendations regarding what to do while awaiting your PCR test (for a more detailed explanation, including definitions of quarantine vs isolation: https://myvirtualphysician.com/2022/01/22/ive-been-exposed-to-covid-19-what-now/)
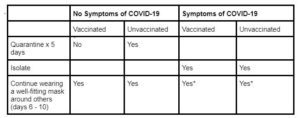
*Continue isolating as long as you are experiencing symptoms of COVID-19 and while awaiting test results. You can end isolation after 5 full days if your symptoms are improving AND you are fever-free for 24 hours (without taking any fever-reducing medications), provided your other symptoms have improved.
* Please contact your medical provider if you test positive for COVID-19 and/or if you are experiencing any severe symptoms of COVID-19.
If you haven't yet been in the situation where you've received 'the call', the one in which someone you were recently in close contact with rings you a day or two later to let you know they tested positive for COVID-19 (these days, this means positive for the highly transmissible Omicron variant), it’s highly likely that you may be in the coming weeks. Both Dr. Janet Woodcock, acting head of the FDA, and Dr. Anthony Fauci, President Biden's chief medical adviser, stated recently that they expect the majority of Americans will eventually be infected with COVID-19. Mercifully, Omicron infection appears to cause less severe disease than previous variants and a large swath of the population has a degree of protective immunity, either through vaccination and/or a previous infection.
The CDC has recently updated its recommendations for quarantine following COVID-19 exposure. To briefly summarize, if you've just found out you had an exposure and you are not experiencing any symptoms (asymptomatic):
- Stay calm. It takes time for the virus to reach detectable levels. The CDC recommends testing on Day 5 (Day 0 is the day of exposure). Testing experts have noted that Omicron tends to replicate more quickly, though, and suggest that–if possible–at-home testing can begin on day three, and continue daily through day seven.
- If you are fully vaccinated and boosted, then you do not need to quarantine (again, if asymptomatic). However, it is recommended to wear a well-fitting mask, such as an N95 or KN95, for 10 days when you are around other people.
- If you are unvaccinated or vaccinated but did not receive a booster, quarantine for 5 days. If you are asymptomatic, you can stop quarantining but should continue to wear a well-fitting mask such as an N95 or KN95 around others for 5 more days.
If you develop symptoms of COVID-19 AND/OR receive a positive test, isolate for at least 5 days if you have confirmed or suspected COVID-19, regardless of vaccination status. Day 0 is counted as either the first day of symptoms or, for those without symptoms, the date of an initial positive COVID-19 test. Isolation means you stay home, separate yourself from others in your household, and – if necessary to be around them -- wear a well-fitting mask at all times. If you had COVID-19 and had symptoms, you can end isolation after 5 full days if you are fever-free for 24 hours (without taking any fever-reducing medications), provided your other symptoms have improved. If you tested positive for COVID-19 but never developed symptoms, you can end isolation after 5 full days. In all cases, you should continue wearing a well-fitting mask around others for 10 days (days 6 - 10).
Serial Testing: If you want to take a test at the end of the 5-day isolation period, it is best to use an antigen test (the more sensitive PCR test can detect very low levels of virus and will often stay positive for weeks after infection). If the test result is negative, as above, end isolation and continue mask-wearing through day 10. If the test result is positive, you should continue isolating until day 10. If you continue to test positive on an antigen test after day 10, the safest route is to continue isolation until you have a negative test result. For many people, this may not be feasible; if this is the case, experts recommend continuing to wear a highly effective mask, such as an N95 or KN95, around others, and being mindful of your contacts (eg avoid public transportation and situations where immunocompromised persons may be present).
* Please check in with your health care provider for concerning symptoms or test results
|